H2CO is an organic compound and is classified as an aldehyde. Andehides are chemicals that have the functional group -HCO- in their molecules and formaldehyde is the lowest member of this group with one carbon atom.
| Join the channel Telegram of the AnonyViet 👉 Link 👈 |
Molecular formaldehyde is a toxic, colorless gas with an unpleasant and pungent odor. In its solid form, it can exist as a trimer (1,3,5-trioxane) or as a polymer known as Paraformaldehyde.
Formaldehyde is usually stored in an aqueous solution called formalin. This chemical, due to its high reactivity, is also one of the most important building blocks of synthetic chemistry.
Properties of Formaldehyde
- It has a molar mass of 30.02 g mol −1 .
- It is a poisonous gas with an unpleasant and pungent odor.
- It has a boiling point of -19°C and a melting point of -92°C.
- It is soluble in acetone as well as in aqueous solution
| Part nameeh | Formaldehyde |
| Chemical formula | H2CO |
| The molecular geometry of H2CO | Flat triangle |
| Electron Geometry of H2CO | Flat triangle |
| hybridization | sp2 |
| Nature | Polar molecule |
| Total valence electrons for H2CO | twelfth |
How to draw lewis structure for H2CO
The structure of H2CO lewis is made up of one oxygen, one carbon and two hydrogen. These atoms are structured in such a way that the carbon atom is held in the central position and it is bonded to two hydrogens by a single bond and a double bond to an oxygen atom.
There are a total of 2 lone pairs and 4 bond pairs (two single bonds + one double bond) present in the lewis structure of H2CO.
Let’s see how to draw lewis dot structure of H2CO with some simple steps.
Follow some steps to draw lewis dot structure of H2CO
Count the total number of valence electrons in H2CO
As we all know, lewis diagram is the valence electron representation of atoms in a molecule. Valence electrons are the outermost electrons of an atom that can participate in bond formation by either donating or accepting.
So in the first step, we will find valence electrons of H2CO. To calculate this, look at the periodic group of carbon, hydrogen, and oxygen.
Carbon atom belongs to group 4A or 14th in the periodic table, so it has 4 valence electrons. The oxygen atom is in Group 6A or 16, so it has 6 valence electrons while the hydrogen atom is in Group 1A, so the number of valence electrons in hydrogen is 1.
⇒Total valence electrons in carbon = 4
⇒Total valence electrons in oxygen = 6
⇒ Total number of valence electrons in hydrogen = 1
∴ Total number of valence electrons available to draw the Lewis structure of H2CO = 4 + 1(2) + 6 = 12 valence electrons [∴H2CO phân tử có một carbon, một oxy và hai hydro]
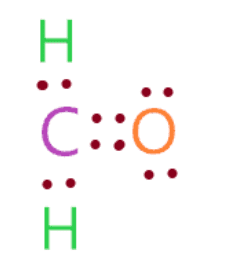
Find the atom with the least electronegativity and place it at the center
This step can be complicated at times but in the case of the H2CO molecule it is easy to identify the least electronegative atoms to place at the center in the lewis diagram.
One should remember that the hydrogen atom can never occupy a central position in the lewis diagram because it can only have at most two valence electrons in the outer shell, so it can never share more than two electrons if needed.
Since the central position requires the atom to tend to share electrons with other atoms. The electronegativity of the oxygen atom is 3.44 and that of the carbon atom is 2.55.
Therefore, place the carbon at central location while spreading oxygen and hydrogen atoms evenly around it.
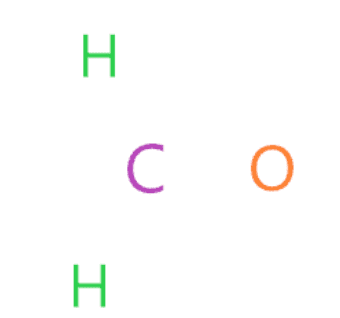
Connect the outermost atom to the central atom by a single bond
In this step, each outer atom (oxygen and hydrogen) is bonded to the central atom (carbon) with the help of a single bond.
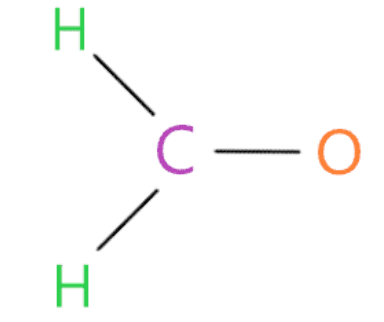
Now we will count how many valence electrons we have used in the above structure. In the above structure, we used three single bonds to connect two hydrogen atoms and one oxygen atom to the central atom (carbon).
A single bond means two electrons, so (3 single bonds × 2) = 6 valence electrons are used out of the total 12 valence electrons available to draw the Lewis structure of H2CO.
∴ (twelfth – 6) = 6 electrons hooha childpoop
So we have 6 more valence electrons left.
Put the remaining valence electrons starting from the outer atom first
We will now start putting the remaining valence electrons into the outer atoms to complete their octet. In an H2CO molecule, the oxygen and hydrogen atoms are the outer atoms.
Hydrogen atom needs only two valence electrons to complete its octet and achieve stability, moreover, oxygen atom needs 8 electrons to complete its octet and is in stable form.
If you look at 3rd step structure both hydrogen atoms have received two electrons in their outer shells in the form of a single bond, so both of these atoms have completed their octet.
So just put the remaining valence electron on the oxygen atom until it completes the octet shell.
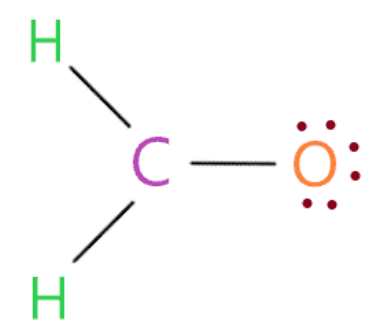
So by looking at the above structure we see that the oxygen atom has 8 electrons (6 electrons represented by a dot + 2 electrons in a single bond connected to it) in its octet shell. Therefore, it also completes its octet.
In the above structure, we used total 12 valence electrons (three single bonds contain 6 electrons and 6 electrons are dots).
Therefore, we now have no valence electrons left.
Complete the central atom octet and make a covalent bond if necessary
The central atom in the H2CO molecule is carbon which also needs 8 electrons in the outer shell to complete the octet. LIVE quaternary structure we see the carbon atom has 6 electrons in the outer shell in the form of three single bonds but it needs 2 more electrons to complete the octet.
But the problem is that we don’t have any valence electrons left, so in this situation we will convert a single pair of oxygen atoms into a single covalent bond.
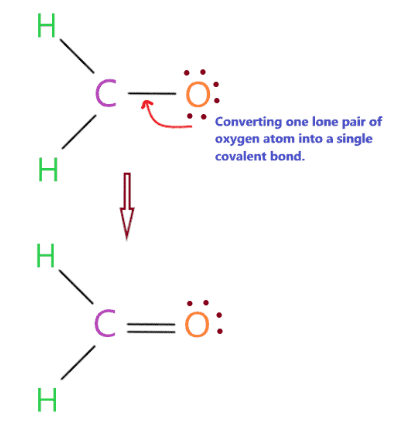
As you can see in the above structure, we have successfully converted a lone pair of oxygen atoms to a covalent bond, so the carbon center has also completed its octet because it has 8 electrons (two bonds). single bond + one double bond) in its valence or outer shell.
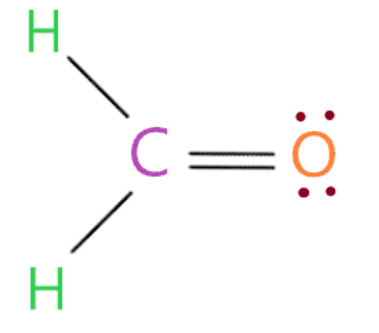
So by looking at the above structure we see that the oxygen atom has 8 electrons (6 electrons represented by a dot + 2 electrons in a single bond connected to it) in its octet shell. Therefore, it also completes its octet.
In the above structure, we have used a total of 12 valence electrons (three single bonds contain 6 electrons and 6 electrons are dots).
Therefore, we now have no valence electrons left.
Check stability with the help of formal charge concept
“The less formal charges on the atoms, the better the stability of the lewis diagram.”
To calculate the formal charge on an atom. Use the formula given below

Let’s start with the central atom of carbon in the H2CO molecule.
For the carbon atom:
⇒ Valence electron of carbon = 4
⇒ Lone electron pair on carbon = 0
⇒ Shared electron pair around carbon (two single bonds and one double bond) = 8
∴ (4 .) – 0 – 8/2) = 0 formal charge on the central carbon atom.
For the oxygen atom
⇒ Number of valence electrons of oxygen = 6
⇒ Single electron pair on oxygen = 4
⇒ Number of shared electron pairs around oxygen (1 double bond) = 4
∴ (6 .) – 4 – 4/2) = o the formal charge of the oxygen atom.
For the hydrogen atom
⇒ Number of valence electrons of hydrogen = 1
⇒ Single electron pair on hydrogen = 0
⇒ Shared electron pair around hydrogen (1 single bond) = 2
∴ (first – 0 – 2/2) = o formal charge on both hydrogen atoms.
So each atom (oxygen, hydrogen and carbon) in the lewis structure H2CO has a formal charge of zero.
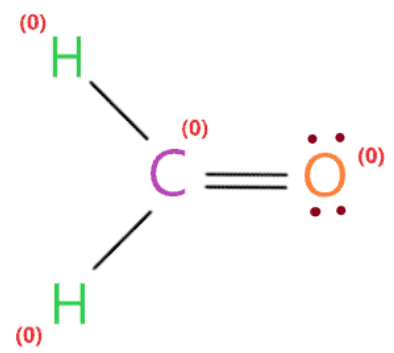
Therefore, above lewis structure of H2CO is the most consistent, reliable and intrinsically stable since the overall formal charge is zero.
What is the electron and molecular geometry of H2CO?
The molecular geometry of H2CO is planar trigonometric because the carbon central atom has no lone pair and is attached to two hydrogen atoms and one oxygen atom with the help of two single bonds and one double bond. So there are three regions of electron density around the central carbon atom.
“An electron density region means the group of bound or unbound electrons present around the atom. A single bond, double bond or even triple bond around the atom counts as a region.”
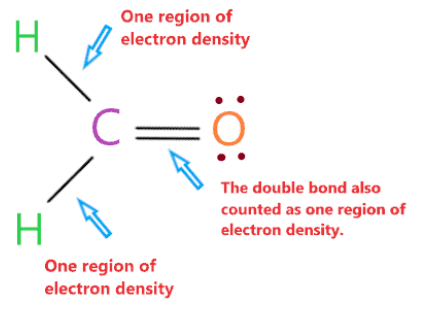
The pair of electrons around the central carbon atom will repel each other and try to move away from each other, they will occupy the position where the repulsion between them becomes the smallest.
According to VSEPR theory, the central atom with three electron density regions uses trigonal planar geometry because the repulsion is the smallest of the electron pairs in this position.
Thus, the final molecular geometry of H2CO appears as a trigonal plane.
The electron shape of H2CO is also trigonal planar since there is no lone pair present on the central atom (carbon), only the bond pair will be counted to determine the shape of H2CO.
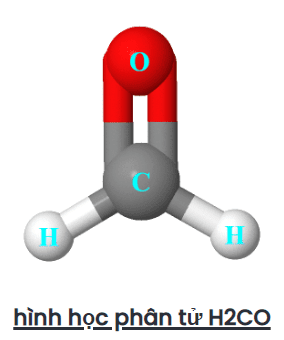
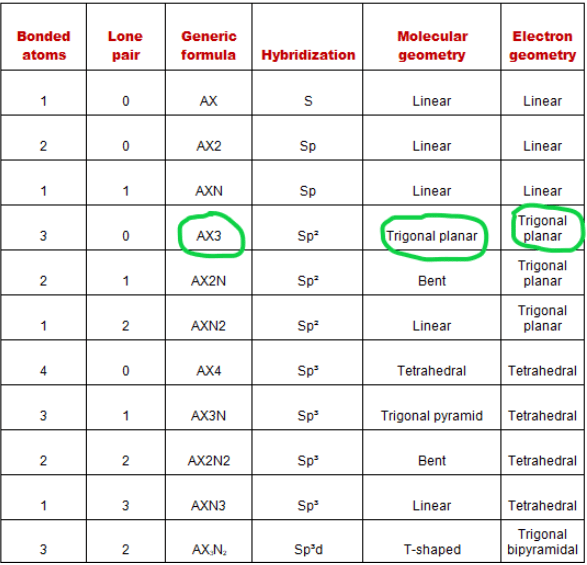
We can also find the electron and molecular shape of H2CO by AXN method and VSEPR plot.
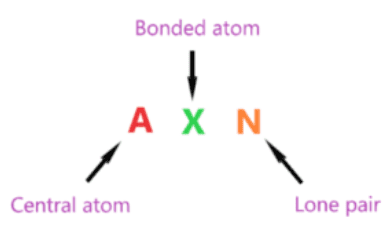
AXN is a simple formula expressing the number of bonded atoms and lone pairs on the central atom to predict the shape of a molecule using a VSEPR plot.
The symbol for AXN of the molecule H2CO:
- A is the central atom, so carbon is the central atom in the H2CO . molecule A = Carbon
- X denotes atoms bonded to the central atom, carbon bonded to two hydrogen atoms and one oxygen atom. Therefore, X = 3
- N represents the lone pair on the central atom, according to the lewis structure of H2CO, the carbon central atom has no lone pairs. Therefore, N = 0
So, the general formula AXN for the H2CO molecule becomes AX 3 WOMEN 0 or AX 3 .
According to the VSEPR diagram, if a molecule has the general formula AX 3 then its molecular geometry will be trigonal plane and electron geometry will also be trigonal plane.
Look at the VSEPR chart below